AlloAid® DIP
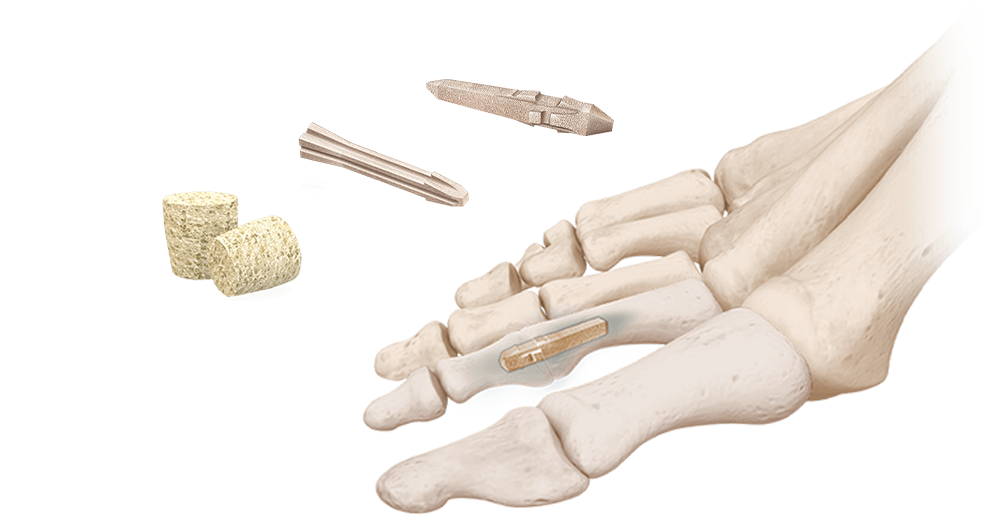
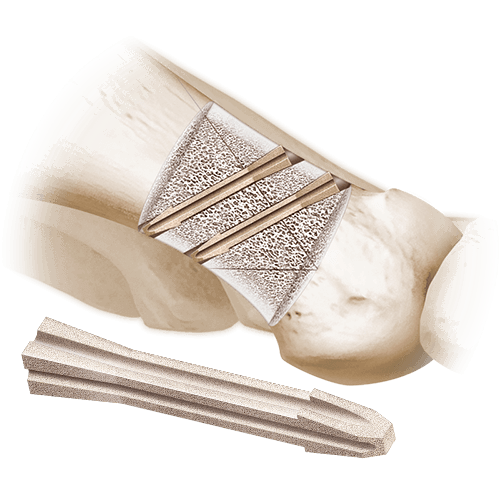
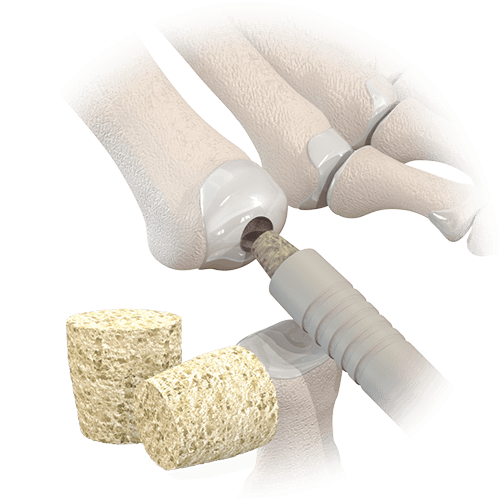
This engineered, cortical bone allograft has osteoconductive properties and is intended for arthrodesis in small bone fusion procedures. The implant is designed with positioning ramps which allows for accurate placement and ensures a press-fit to mitigate the risk of subsidence. The cross-sectional shape design resists rotation, while tapered ends assist with the ease of insertion.
AlloAid® DIP is available in two sizes, and is accompanied by a convenient sterile, disposable instrument set that includes laser-marked reamers and bowed-nose forceps. No post-op hardware removal is required. AlloAid® DIP complies with all FDA, AATB, and State Regulatory requirements for donor screening, recovery, and testing.