Release Date: August 12, 2025
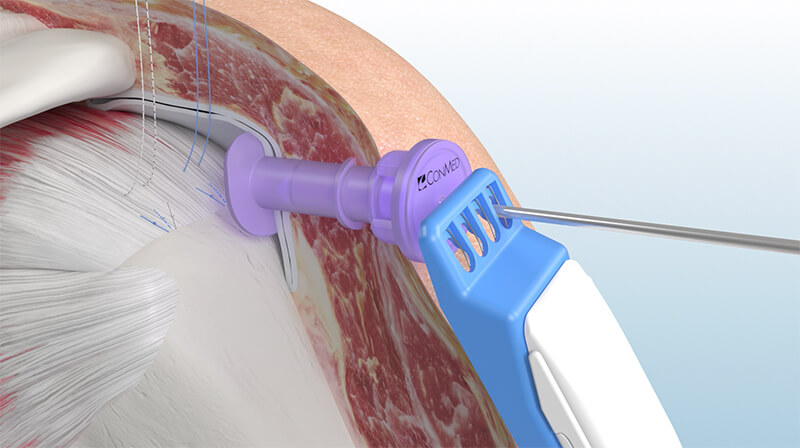
CONMED Launches BioBrace®RC to Revolutionize Rotator Cuff Repair Surgeries
Proprietary arthroscopic delivery system designed to streamline surgical augmentation and enhance procedural performance using the FDA-cleared BioBrace® implant.