The Problem
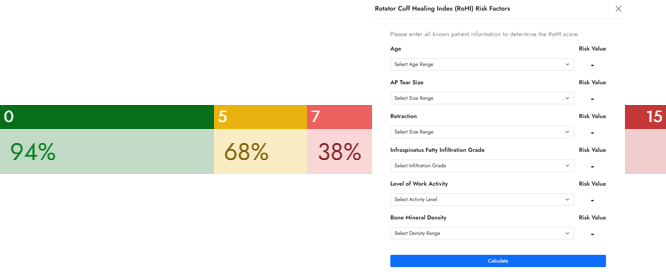
RoHI (Rotator Cuff Healing Index)
Since numerous factors impact the risk of failure in tendon healing, the RoHI scoring index was created to help predict rotator cuff healing.

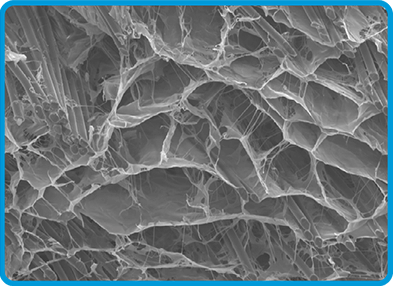

BioBrace® Porous Structure
42% Increase
In ACL Graft Volume14
BioBrace® Filled With New Tissue
180% Increase
In Rotator Cuff Tendon Thickness14
Rotator Cuff Augmentation Technique Videos



Get It in Writing
Our written surgical technique guide outlines simple, efficient ways to augment with BioBrace®. This includes on-lay augmentation of a single row repair, integrated augmentation of a double row repair, and more.
Related Products
1 Park JY, Lee JH, Oh KS, Chung SW, Choi Y, Yoon WY, Kim DW. Rotator cuff retear after repair surgery: comparison between experienced and inexperienced surgeons. Clin Shoulder Elb. 2021 Sep;24(3):135-140. doi: 10.5397/cise.2021.00073. Epub 2021 Sep 1. PMID: 34488293; PMCID: PMC8423529.
2 Rashid, M, C Cooper, J Cook, D Cooper, S Dakin, S Snelling, and A Carr. “Increasing Age and Tear Size Reduce Rotator Cuff Repair Healing Rate at 1 Year.” Acta Orthopaedica 88, no. 6 (2017): 606–11. https://doi.org/10.10 80/17453674.2017.1370844.
3 Neri, B, K Chan, and Y Kwon. “Management of Massive and Irreparable Rotator Cuff Tears.” Journal of Shoulder and Elbow Surgery 18, no. 5 (2009): 808–18. https://doi.org/10.1016/j.jse.2009.03.013.
4 McCarron, J. A., Derwin, K. A., Bey, M. J., Polster, J. M., Schils, J. P., Ricchetti, E. T., & Iannotti, J. P. (2013). Failure with continuity in rotator cuff repair “healing”. The American journal of sports medicine, 41(1), 134–141. https://doi.org/10.1177/0363546512459477
5 Iannotti, J. P., Deutsch, A., Green, A., Rudicel, S., Christensen, J., Marraffino, S., & Rodeo, S. (2013). Time to failure after rotator cuff repair: a prospective imaging study. The Journal of bone and joint surgery. American volume, 95(11), 965–971. https://doi.org/10.2106/JBJS.L.00708
6 Ponce, B. A., Hosemann, C. D., Raghava, P., Tate, J. P., Sheppard, E. D., & Eberhardt, A. W. (2013). A biomechanical analysis of controllable intraoperative variables affecting the strength of rotator cuff repairs at the suture-tendon interface. The American journal of sports medicine, 41(10), 2256–2261. https://doi.org/10.1177/0363546513499228
7 Longo, U.G., Carnevale, A., Piergentili, I. et al. Retear rates after rotator cuff surgery: a systematic review and meta-analysis. BMC Musculoskelet Disord 22, 749 (2021). https://doi.org/10.1186/s12891-021-04634-6
23. https://acltear.info/acl-reinjury
8 Bushnell, B. D., Connor, P. M., Harris, H. W., Ho, C. P., Trenhaile, S. W., & Abrams, J. S. (2022). Two-year outcomes with a bioinductive collagen implant used in augmentation of arthroscopic repair of full-thickness rotator cuff tears: final results of a prospective multicenter study. Journal of shoulder and elbow surgery, 31(12), 2532–2541. https://doi.org/10.1016/j. jse.2022.05.025
9 Zhang, T., Ajayi, A., Hajjar, M., Fleckenstein, C. M., Nolan, J., & Hasan, S. S. (2023). Arthroscopic Repair of Retracted Large and Massive Rotator Cuff Tears with and without Augmentation with a Bio-Inductive Collagen Implant Reveals Substantial and Comparable Clinical Improvement. Arthroscopy: the journal of arthroscopic & related surgery, S0749-8063(23)00868-X. Advance online publication. 1.https://doi.org/10.1016/j.atro.2023.10.024
10 Snyder, S. J., Arnoczky, S. P., Bond, J. L., & Dopirak, R. (2009). Histologic evaluation of a biopsy specimen obtained 3 months after rotator cuff augmentation with GraftJacket Matrix. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association, 25(3), 329–333. https://doi.org/10.1016/j.arthro.2008.05.023
11 Carter, AJ, V Lovric, P Morberg, J Ott, J Bendigo, J Komenda, M Aronson, K Rocco, S Arnoczky, and WR Walsh. 2021. “Characterization of a Novel BioInductive Biocomposite Scaffold for Tendon and Ligament Healing.” Presented at the Orthopaedic Research Society (ORS) 2021 Annual Meeting; February 12-16, 2021, Virtual.
12 Walsh, WR, AJ Carter, V Lovric, J Crowley, D Wills, T Wang, G Kanski, R Stanton, S Arnoczky, and R Arciero. 2021. “TissueEngineered Augmentation of A Rotator Cuff Tendon Using A Novel Bio-Inductive Biocomposite Scaffold: A Preliminary Study In Sheep.” Presented at the Orthopaedic Research Society (ORS) 2021 Annual Meeting; February 12-16, 2021, Virtual.
13 Based on internal test data.
14 Based on preclinical animal data.