MINIMIZE Migration, MAXIMIZE Control
GORE® VIABIL® is proven to minimize the risk of reintervention1
GORE® VIABIL® is proven to minimize the risk of reintervention1
GORE® VIABIL® Biliary Endoprosthesis is the non-foreshortening fully covered metal stent in the market and remains in the same location throughout deployment, eliminating the need to use a push-pull technique. Physicians can trust that the length and position of the stent will be the same pre-deployment and post-deployment.
Patency, or the ability for a stent to remain open and unoccluded, is a crucial characteristic of any Self-Expanding Metal Stent (SEMS).
The GORE® VIABIL® Biliary Endoprosthesis maintains higher primary patency than the leading stent at 3, 6, and 12 months post-deployment2,3 due to its moderate radial force, low axial force, and durable, nonporous ePTFE/FEP liner.
The optimal balance of low Axial force (Af) and moderate Radial force (Rf), allowing natural conformance of the stent to the bile duct anatomy while maintaining industry-leading primary patency rates.
Optimal Balance of Force: The preferred combination of low Af and moderate Rf to minimize risk of migration, conforming naturally to the bile duct anatomy.4
Low Axial Force: Reduces migration, kinking, sludge formation, and cholangitis.5
Malignant biliary stricture migration rate comparison1
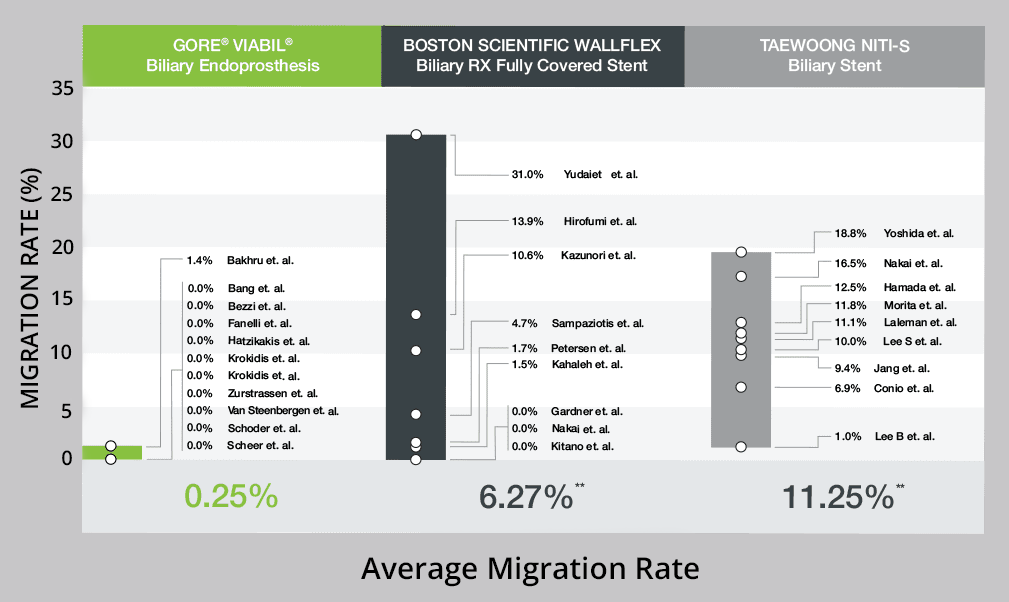
Malignant biliary stricture migration rate comparison1
*Not available in all markets.
Header Illustration: Keith Kasnot, MA, CMI, FAMI
* If deployed as instructed, the endoprosthesis will not appreciably foreshorten. Data on File.
** p<0.00000001, when compared to GORE® VIABIL® Biliary Endoprosthesis migration rates.
1 W. L. Gore & Associates, Inc; Biliary Fully Covered Metal Stents Systematic Review of the Clinical Literature. Flagstaff, AZ; 2019. [Work plan]. WP111272.
2 Krokidis M, Fanelli F, Orgera G, Bezzi M, Passariello R, Hatzidakis A. Percutaneous treatment of malignant jaundice due to extrahepatic cholangiocarcinoma: covered Viabil stent versus uncovered Wallstents. Cardiovascular & Interventional Radiology 2010;33(1):97-106.
3 Kitano M, Yamashita Y, Tanaka K, et al. Covered self-expandable metal stents with an anti-migration system improve patency duration without increased complications compared with uncovered stents for distal biliary obstruction caused by pancreatic carcinoma: a randomized multicenter trial. Am J Gastroenterol. 2013 Nov;108(11):1713-22.
4 Isayama H, Nakai Y, Toyokawa Y, et al. Measurement of radial and axial forces of biliary self-expandable metallic stents. Gastrointestinal Endoscopy 2009;70(1):37-44.
5 Isayama H, Mukai T, Itoi T, et al. Comparison of partially covered nitinol stents with partially covered stainless stents as a historical control in a multicenter study of distal malignant biliary obstruction: the WATCH study. Gastrointestinal Endoscopy 2012;76(1):84-92.
6 W. L. Gore & Associates, Inc; Radial Force and Bend Stiffness Characterization of Biliary Stents. Flagstaff, AZ; 2012. [Work plan]. WP103837.