The Association for the Advancement of Medical Instrumentation (AAMI) has published their latest set of standards (TIR99) on proper processing of dilators and ultrasound probes in health care facilities. Find out what standards have changed and how CONMED’s CleanGuide™ stacks up to these standards below.
Changes to AAMI Standards
The latest iteration of AAMI standards is also known as “AAMI TIR99: 2024 Processing of dilators, transesophageal and ultrasound probes in health care facilities”.
Here are some callouts from the new set of standards:
Callout 1:
All dilators and associated equipment should be visually inspected for cleanliness and integrity after each use and before disinfection or sterilization. Lighted magnification is recommended.
Callout 2:
Workflow should be unidirectional from the processing area and then on to storage or distribution. The space should accommodate an instrument cleaning sink, a preparation surface, quality monitors, chemicals, record keeping supplies, hand hygiene station including a handwash sink, waste disposal, required utilities.
Callout 3:
Dilators are semi-critical devices: These devices should be thoroughly cleaned and then processed by sterilization.
How CleanGuide™ Disposable OTW Esophageal Dilators Lives Up to These Standards
If you already use CleanGuide™ in your practice, we have good news:
CleanGuide™ is already fully compliant to these new standards.
With CleanGuide™, you can use what you need, when you need it, and discard after—keeping the entire process sterile.
Moreover,
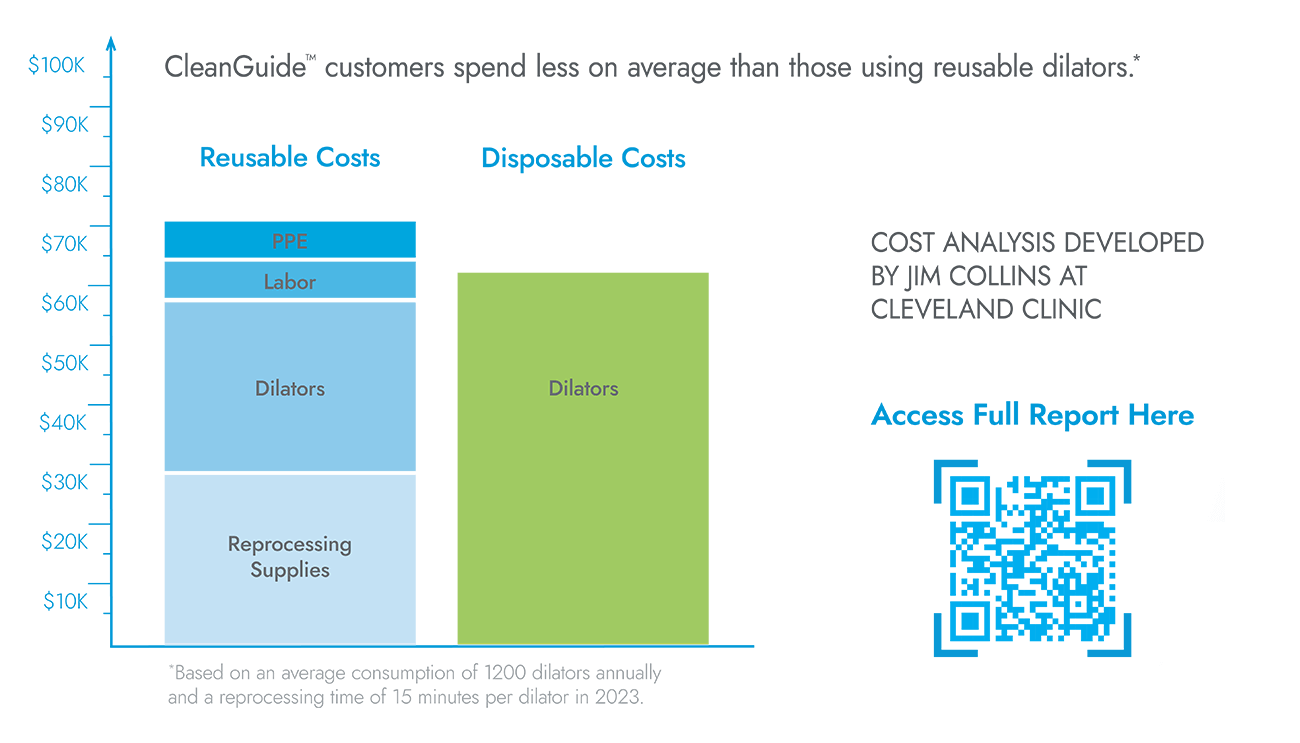
CleanGuide™ Dilators could help save you and your practice money.
According to a cost savings study, CleanGuide™ customers spend less on average than those using reusable dilators.