There have been numerous studies over the past two decades comparing biliary stents. With all biliary stents, there is a chance of adverse events happening.
Stent occlusion is common in patients with biliary stents for malignant obstruction. Stent occlusion may be caused by tumor ingrowth, tumor overgrowth, and luminal impaction from biliary sludge.1 Patients with stent occlusion or stent migration typically undergo a repeat ERCP procedure in which the stent is either removed or replaced.1
The typical indications for biliary stenting are palliative biliary drainage for patients with biliary obstruction from surgically incurable cancer, and preoperative biliary drainage for patients with resectable tumors who require timely biliary decompression.1 A main area of concern in choosing a stent comes down to wanting to give these patients the highest quality of life. This includes choosing a biliary stent that can help minimize the patients worry of stent occlusion or stent migration.
In a recent webinar, Dr. Rawad Mounzer from Arizona discusses some clinical efficacy studies that feature the GORE® VIABIL® Biliary Endoprosthesis.
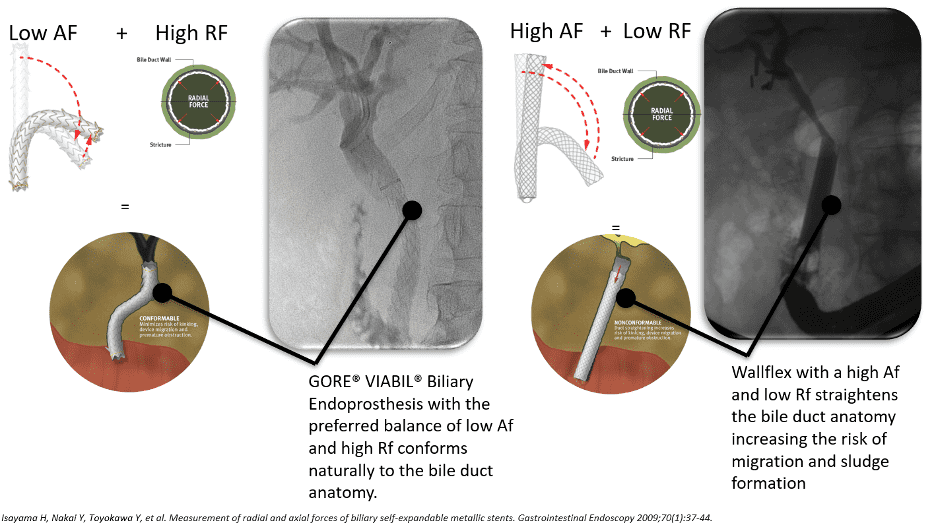
Dr. Mouzner explains, “When this stent is compared to the Boston Scientific WallFlex stent in regard to axial and radial forces, you can see that the GORE® VIABIL® stent has twice the radial force and 1/10th the axial force.”
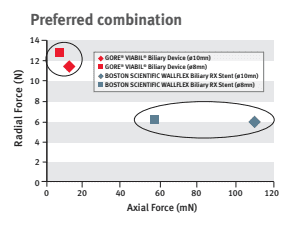
This optimal balance of low axial force and moderate radial force allows natural conformance of the stent to the bile duct anatomy to minimize risk of migration.3 A high axial force does not conform as well to curved biliary anatomy, which can increase the risk of stent migration.4
Dr. Mouzner also comments on a malignant biliary stricture migration rate comparison based on 25 scientific publications between 2002 and 2018. “The GORE® VIABIL® Fully Covered Metal Stent has a significantly reduced risk of migration when compared to some of the competitors, including the Boston Scientific WallFlex and the Taewoong stent.”
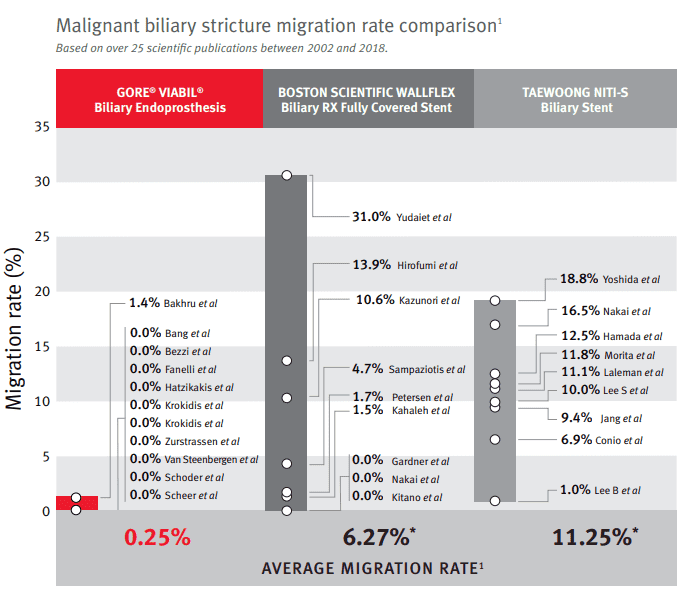
This 25X reduction in migration rate is significant. Proprietary built-in anti-migration fins on the GORE® VIABIL® gently stabilize the device within the common bile duct, resisting the anatomical forces that could push the device out of place.
In addition, GORE® VIABIL® features higher primary patency rates than the competition. A crucial characteristic of any self-expanding metal stent, patency is the ability for a stent to remain open and unoccluded. Clinical publications show the VIABIL maintains higher primary patency than the leading competitor at 3, 6, and 12 months post-deployment, when implanted to manage malignant biliary strictures.6,7 VIABIL’s moderate radial force and low axial force, along with a durable nonporous ePTFE/FEP liner, are designed to enhance patency.
To view Dr. Mounzer’s full presentation, click here.
To learn more about GORE® VIABIL®, click here.
1 Adler, D.G., & Byrne, K. R. (2024, January 2). Endoscopic stenting for malignant biliary obstruction. UpToDate. Version 39.0. https://www.uptodate.com/contents/endoscopic-stenting-for-malignant-biliary-obstruction#:~:text=Adverse%20events%20%E2%80%93%20Common%20complications%20of,cholangitis%2C%20perforation%2C%20and%20bleeding.
2 W. L. Gore & Associates, Inc; Biliary Fully Covered Metal Stents Systematic Review of the Clinical Literature. Flagstaff, AZ; 2019. [Work plan]. WP111272. p<0.00000001, when compared to GORE® VIABIL® Biliary Endoprosthesis migration rates.
3 Isayama H, Nakai Y, Toyokawa Y, et al. Measurement of radial and axial forces of biliary self-expandable metallic stents. Gastrointestinal Endoscopy 2009;70(1):37-44.
4 Isayama H, Mukai T, Itoi T, et al. Comparison of partially covered nitinol stents with partially covered stainless stents as a historical control in a multicenter study of distal malignant biliary obstruction: the WATCH study. Gastrointestinal Endoscopy 2012;76(1):84-92.
5 W. L. Gore & Associates, Inc; Radial Force and Bend Stiffness Characterization of Biliary Stents. Flagstaff, AZ; 2012. [Work plan]. WP103837.
6 Krokidis M, Fanelli F, Orgera G, Bezzi M, Passariello R, Hatzidakis A. Percutaneous treatment of malignant jaundice due to extrahepatic cholangiocarcinoma: covered Viabil stent versus uncovered Wallstents. Cardiovascular & Interventional Radiology 2010;33(1):97-106.
7 Kitano M, Yamashita Y, Tanaka K, et al. Covered self-expandable metal stents with an anti-migration system improve patency duration without increased complications compared with uncovered stents for distal biliary obstruction caused by pancreatic carcinoma: a randomized multicenter trial. Am J Gastroenterol. 2013 Nov;108(11):1713-22.